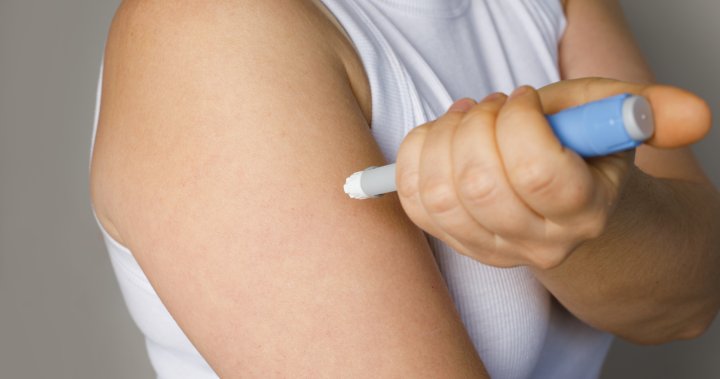
Well being Canada is recalling compounded medication containing semaglutide, which is a key part in common drugs like Ozempic and Wegovy.
The medication have been compounded by Alberta-based Create Compounding Pharmacy and comprised semaglutide, which belongs to the GLP-1 class of medicine, and pyridoxine, which is a type of vitamin B6.
“The product was produced with an unauthorized energetic pharmaceutical ingredient,” Health Canada said in the recall published Tuesday.
The medication have been offered in syringe or vial type.
Compounding is when pharmacies or different practitioners have the substances wanted to combine and put together specialty drugs, and accomplish that. It was regularly utilized in Canada by pharmacies throughout the COVID-19 pandemic when manufactured provides of youngsters’s ache drugs have been in brief provide however the pharmaceutical elements to combine these drugs have been nonetheless out there in some pharmacies.
It is just meant to be executed in instances the place there may be restricted provide of a medicine “and shouldn’t be executed solely for financial causes for the healthcare professionals,” according to Health Canada guidelines on compounding.

Ozempic maker Novo Nordisk says it’s the one firm in Canada with Well being Canada-approved merchandise containing semaglutide.

Get weekly well being information
Obtain the most recent medical information and well being data delivered to you each Sunday.
Novo Nordisk told Global News last summer that it had filed a grievance with Well being Canada relating to the promotion and sale of compounded semaglutide.
“We’re conscious that a number of compounding pharmacies, weight reduction clinics, and medical spas are purporting to promote or supply unapproved compounded semaglutide merchandise each in Canada and the U.S.,” a spokesperson for Novo Nordisk Canada mentioned in an emailed assertion in July 2024.
Well being Canada is advising sufferers to seek the advice of their physician earlier than stopping using the recalled medication or for any well being considerations.
Customers must also contact the corporate if they’ve any questions in regards to the recall, the company mentioned.
Any uncomfortable side effects could be reported to Well being Canada.
Final yr, the U.S. Meals and Drug Administration (FDA) issued an alert warning docs and sufferers about dosing errors involving compounded semaglutide injectable merchandise that have been allotted in multiple-dose vials.
The well being regulator mentioned it had acquired reviews of opposed occasions, with some requiring hospitalization, that could be associated to overdoses on account of sufferers incorrectly self-administering the compounded drug and health-care suppliers miscalculating doses.
Due to the dosing errors, sufferers mistakenly took 5 to twenty occasions greater than the meant dose, the FDA mentioned.

The Novo Nordisk spokesperson advised World Information on the time that the FDA alert doesn’t relate to the corporate’s merchandise, Ozempic or Wegovy, however pertains to compounded semaglutide merchandise, which aren’t permitted in Canada nor the U.S.
Among the many opposed occasions reported within the U.S., some sufferers skilled nausea, vomiting, stomach ache, fainting, headache, migraine, dehydration, acute pancreatitis and gallstones.
Semaglutide works by serving to management blood sugar ranges and triggering a sense of fullness.
In recent times, Canada, like different international locations, has seen a excessive demand for drugs like Ozempic, which is primarily permitted for the remedy of Sort 2 diabetes however has been used off-label for weight reduction.
Wegovy, an on-label weight reduction remedy containing the identical drug as Ozempic however at the next formulation and made by the identical producer, also became available to Canadians last year.
–with recordsdata from Reuters
© 2025 World Information, a division of Corus Leisure Inc.
Source link