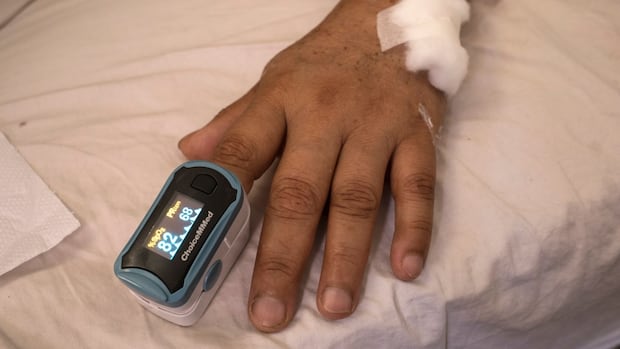
The U.S. Meals and Drug Administration proposed new steering for units used to watch blood oxygen ranges to enhance their accuracy and efficiency throughout a variety of pores and skin tones.
FDA says suggestions deal with accuracy considerations of medical units affected by elements like pores and skin tone

The U.S. Meals and Drug Administration on Monday proposed new steering for units used to watch blood oxygen ranges to enhance their accuracy and efficiency throughout a variety of pores and skin tones.
The updated guidance recommends that producers collect scientific information to judge system efficiency accuracy throughout a variety of pores and skin pigmentations and improve the variety of individuals on whom these units are examined.
“Our draft suggestions are primarily based on the perfect accessible science to assist deal with considerations of disparate efficiency of pulse oximeters primarily based on a person’s pores and skin pigmentation,” stated Michelle Tarver, director of the FDA’s Middle for Units and Radiological Well being.
The regulator stated the steering, if finalized, would apply to oxygen monitoring units often called pulse oximeters that are used for medical functions.
It might not apply to the units which might be offered for normal wellness and should not reviewed by the company.
Source link